Application Example
Kinetics of Steel Refining in a Ladle Furnace
After steelmaking, which is mostly performed in a basic oxygen furnace (BOF) or electric arc furnace (EAF), steel is usually tapped into a ladle where certain additions are made (deoxidation agents, slag formers, certain alloying elements) and then transferred to the ladle furnace (LF). The LF fulfils many purposes in the steel refining process, including removal of unwanted non-metallic phases and volatile elements.
The reactions taking place in a ladle furnace are a complex interplay between equilibrium thermodynamics that define the direction of chemical reactions, and kinetics that define how fast the equilibrium state is approached.
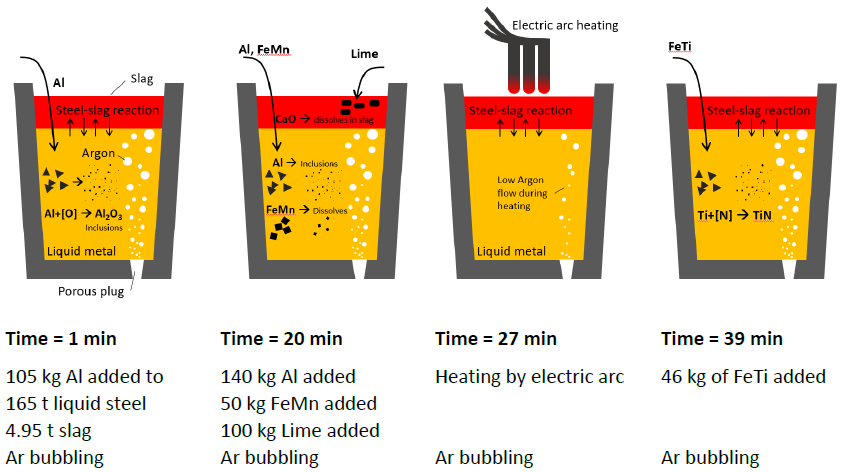
An overview of the LF process that is simulated in this example. Each ladle represents one time-step in the process when additions are made or heat or cooling is applied to refine the steel.
In this example, a full kinetic simulation of the LF refining process schematically shown in the image above is set up using Thermo-Calc’s Process Metallurgy Module. The simulation is based on an LF process described in a publication by K. J. Graham and G.A. Irons (2009) “Toward Integrated Ladle Metallurgy Control.” Iron and Steel Technology. 6(1): 164-173.
The results of this simulation are compared to the experimentally determined steel, slag, and inclusion composition as a function of processing time.