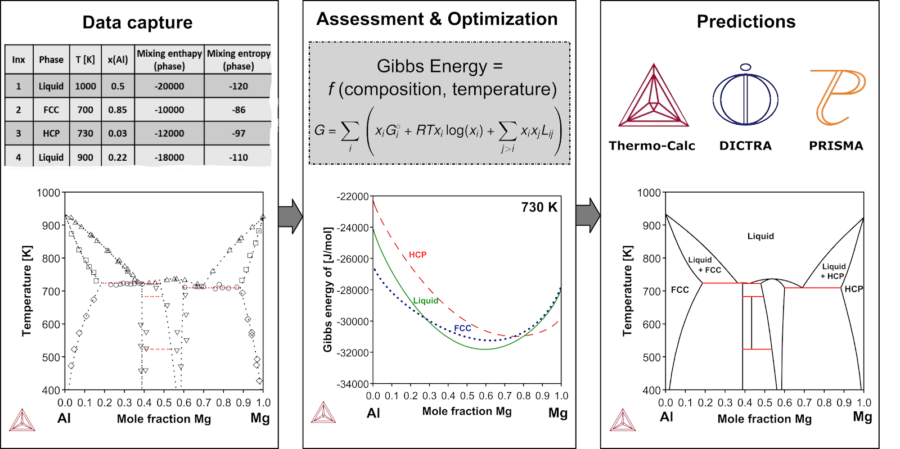
Data Capture
The first step of CALPHAD modeling is to collect experimental data on the materials system of interest. In the case of thermodynamics, high quality experimental data on phase equilibria, thermochemical properties like enthalpy of mixing or formation energies and crystal structures are the essence of CALPHAD modeling.
In cases where we lack experimental data, we perform ab-initio calculations (or use previously calculated ab-initio values). We also make use of machine learning, empirical relations, and/or rule of thumb to estimate the model parameters for systems with little or uncertain data.
Critical Assessment and Model Selection
The next step in the CALPHAD process is the critical assessment and pre-processing of the collected data. This can also be referred to as Model Selection. For instance, in the case of a phase diagram assessment of a given thermodynamic system with elements A and B, there exists a number of thermodynamically distinct regions or so-called phases. For each of these phases, a model for the Gibbs energy is assigned by a human expert depending on its crystal structure. The Gibbs energy is a polynomial in the chemical composition and temperature (and pressure if it is not fixed in the experiments) of a material, and it is the crucial quantity in computational thermodynamics because it describes all the thermodynamic properties of a material.
This process is similar for all types of properties we include in our databases, but the models may vary. For instance, the Gibbs energy model is used for describing the thermodynamics of a system, while other models are used for describing the thermophysical properties, for example the surface tension of liquids has been described using the modified-Guggenheim model.
Read a detailed description of how we capture and assess thermodynamic data for our thermodynamic and properties databases.
Assessment of Thermodynamic Data
Optimization
After assigning the models to phases, the free parameters of the model are fitted to the input data that was collected in the first step. This step is called optimization and demands extensive human judgement at different stages, mainly due to the fact that the free parameters of all phases should be consistent with each other. In other words, our modeling task is a multi-objective optimization with constraints. This is analogous to training multiple-models in the machine learning context.
In Thermo-Calc, optimization is completed using the built-in PARROT Module, which is available in all Thermo-Calc and Diffusion Module (DICTRA) installations.
Read about the PARROT Module
Storage
Once the parameters for all phases are fitted to the experimental data, the Gibbs energy functions with their optimized free parameters are stored in a text file, or so-called database, with a format that is readable by Thermo-Calc.
Validation
The final step in the process of developing a CALPHAD database is to validate the predictions against experimental results. When developing a multicomponent database for a specific alloy system, validation against data (not used during the optimization of the individual subsystems) from commercial and other multicomponent alloys is critical. If the agreement with real multicomponent commercial alloys is not good for key data points, a re-optimization of one or more lower order systems is the only way to rectify this.
CALPHAD Calculations and Predictions
Once all of the steps are completed and the predictions have been validated, the models, with their fitted parameters, known as databases, are loaded into our computational package, Thermo-Calc, to calculate thermodynamic, kinetic, and other properties of the systems of interest. Typically, we optimize only binary and ternary systems and not for higher-order systems. This is in fact the power of the CALPHAD methodology and the reason is that the models have a physical basis and all the model parameters are intrinsically consistent with each other. Therefore, the extrapolation from binary and ternary systems to higher-order systems works very well. This is ensured by critical validation of the optimized model parameters against the experimental data for higher-order systems.
A Summary of the Process
A summary of the assessment process is illustrated in Figure 2 within the context of a thermodynamic assessment.